Chromatography Techniques | All you need to Know
Chromatography techniques can be classified on the basis of
physical characteristics of stationary and moving phases. The stationary phase
can be solid or liquid and the moving phase can be liquid or gas. When the
steady phase is solid, then this technique is called Adsorption Chromatography.
- Thin-Layer Chromatography or TLC
- Ion-Exchange Chromatography
- Gas-Solid Chromatography or GSC
On the other hand, when the phase constant is fluid, it is
called Partition Chromatography.
It is of two types:
·
Paper Chromatography Techniques
·
Liquid Chromatography or GLC Techniques
In the separation, the liquid mobile phase moves downward,
then descending paper chromatography and if the fluid moving phase moves
upward, it is called ascending paper chromatography.
Column Chromatography Techniques
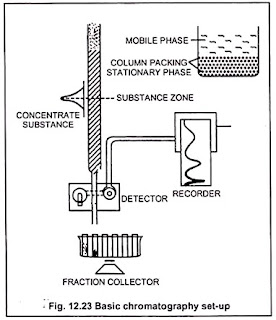
In column chromatography the stationary phase is filled in a
glass or metal tube (column) and the moving phase is driven by this tube. Tube
Diameter generally taken as 20-30 cm. This tube contains up to 50-100 grams of
adsorbent, which can prevent many grams of adsorbate.
Cotton or Glass Wool is used to support the adsorbent. The
sample is dissolved in a small amount of the moving phase and released from the
top of the tube. The sample and moving phase have to run slowly but steadily in
the tube. Different substances present in the sample move at different rates in
the tube. These substances, when coloured, are seen coming out one by one at
the end of the tube, which can be collected as per specified by technique.
Rate of Movement of Substances
Fixed volume (Vs) of fixed phase fill in the tube or column.
The time taken by the volume “Vr” of the moving phase to flow through the tube
is called Retention Time “Tr”. The volume “Vr” is also called the Retention
Volume of the moving phase.
Let us represent the rate of movement of the moving phase
with “h”. The time it taken for a substance to come out of the tube is called
its holding time (TR). During this period (TR), the volume of the moving phase
flows out is called its holding volume (VR).
The rate of movement of a substance is expressed by H. If
the Rf part (Fraction) of a substance is in moving phase, its I-Rf part will be
in steady phase.
If there is an intense exchange of material between the two
phases, then the substance in the Rf part of the period (T) of the experiment
will be in the moving phase and the substance in the T (I-Rf) part will be in
the steady phase. Hence it moves in T period. In T period its speed will be Rf
times the rate of movement of the moving phase. Thus the rate of movement of
material will be H.
H = hRf …….(i)
The rate of motion of a substance will be equal to the rate
of motion of the moving phase and the properties of the fraction of the
substance present in the moving phase. This is the main rule, which applies to
all chromatographic methods –
Rf = H / h = Speed of material / Speed of moving phase
Rf depends on the partition coefficient K between the
stationary and moving phases of matter and the volume of phases Vr and Vs.
Rf = Vr / KVs + Vr ……(ii)
H = hVr / KVs + Vr ……(iii)
Therefore, we can say that the speed of sampling does not
depend on the volume of the sample or the absolute volume of both phases. It is
determined by the ratio between the rate of movement of the moving phase and
multiplication of the partition coefficient of the substances and the volume
between the two phases.
Equation (iii) can also be written as follows:
Vrh / H = KVs + Vr
Since the holding volume is inversely proportional to the
rate of movement:
Vrh / H = VR
Also VR is the holding volume of matter,
VR = KVs + Vr ……(iv)
Hence it is clear that to separate the two substances it is
necessary that their holding volume (VR) must be different. For this, we select
the fixed and moving phases in such a way that the values of the distribution
coefficient (Kd) of the substances vary.
Gel Permeation Chromatography
In gel permeation chromatography, the separation of
molecules is done on the basis of their measurements, size and molecular
weight. This technique is also called molecular sieve or molecular exclusion
chromatography. Its Apparatus is made up of a column which is filled with
spongy-like gel beds.
A solution containing different size molecules is applied to
the mixing column and eluted in the buffer. Large molecules do not move through
the pores of the gel, thus they move rapidly. Whereas small molecules enter the
gel beds and leave behind which come out slowly.
Molecules can be separated by selection of gel beads with
different concentration. Commercially available gel seeds are Cephadex (G-10,
G-25, G-100) Bio Gel (P-10, P-30, P-100) and Cipherose (6B, 4B, 2B).
Gel permeation chromatography can be used in determining
molecular weight. This is done by using columns calibrated with known molecular
weight materials.
Ion exchange chromatography
In ion exchange chromatography, the separation of molecules
is done based on their electric charges. Ion exchange chromatography is a
process in which the free moving ions (called ion exchangers) of a solid are
exchanged with different ions with the same charge present in the solution.
Ion-exchange resin-cation exchanger and anion exchanger are
used for this purpose. A cation exchanger (R + A–) exchanges ion anions (A–)
with other anions (B–) in the solution.
The ion exchanger (which is saturated with one type of ion)
is used as a steady phase. The moving phase is mostly a solution that contains
the same ions from which the ion exchanger is saturated.
When the sample ions are transferred to the column, they
displace some of the original ions, but the ions themselves remain on the
exchanger until they are successively displaced by the ions. Thus they move in
the column.
The rate of movement of a material depends on the degree of
ionization of the substance. Ion exchange separation is mainly done by filling
the ion exchanger in a column.
There are two types of ion exchangers:
·
anion exchangers
·
cation exchangers
Cation Exchanger consists of groups with negative charge and
attracts molecules with cation charge. Since their negative charge is due to
the proteolysis of acidic groups, they are called acidic ion exchange
substances.
Anion Exchanger consists of positive charge groups that
attract molecules with negative charge. Since their positive charge is due to
the combination of protons with alkaline groups, they are also called alkaline
ion exchange substances.
Ion exchange chromatography is used in quantification of
amino acids, purification of proteins, and base composition of nucleotides.
Affinity Chromatography
The principle of fraternity chromatography is based on the
specific and covalent binding properties of other protein proteins called
ligands, for example the enzymes bind to specific ligands, such as the
substrate or reactor.
Ligands are used in this technique, which are joined to the
inert and porous matrix in the column by covalent bonding. Immobilized ligands
act to selectively select the desired protein, while the remaining proteins
pass through the column. The desired protein is absorbed by the ligand.
This can be elicited using the free ligand molecule.
Alternately some reactants that can break the interaction of the protein
ligand. They can also be used in this separation. Fraternal chromatography is
used in the treatment of enzymes, vitamins, nucleic acids, drugs, hormone
receptors, antibodies, etc.
High Performance Liquid Chromatography HPLC:
Read it Here
Gas Chromatography
It is a special method for the dissociation (volatilization)
of volatile materials or volatiles of some volatile matter. The GLC consists of
stationary phase A and solids.
(Diassemaceous Earth or Powered Fire brick) which is
impregnated with evaporative fluid (silicon or polyethylene glycol). It is
packed in a narrow column and controlled at high temperature (200 ° C).
A mixture of volatiles is injected into the column with a
mobile phase, which is inert gas (argon, helium, and nitrogen). Separation of volatile
mixtures depends on the division of components with stationary phase (liquid)
and mobile phase (gas).
Therefore, it is called gas liquid chromatography.
Separation compounds can be identified and determined by dictators. The
detector works on the principle of thermal conductivity.
The gas chromatography was presented by Martin and Synge in
1952. Under this, several methods of analysis of volatile and non volatile
materials are included. It is a highly sensitive technique that has very high
resolution.
Under this technique, volatile matter is injected into a
column filled with a liquid or solid phase. An inert gas, such as nitrogen,
helium, or argon, is flown into the column as a moving phase.
The separation of different components of a volatile
substance is due to the variation of the Partition or Adsorption Coefficient of
the different components of the substance.
The sample is injected through a rubber septum into the
injection port from where it evaporates and enters the column. The column is
placed in an oven which is kept high so that the separating material
evaporates.
Different components of the sample separate and exit the
column with effluent, which are then collected and collected through a
detector. The detector is connected to a recorder.
Different types of detectors are used for different
purposes, such as Thermal Conductivity Detector or TCD, Flame Ionization
Detector or FID, Electron Capture Detector or ECD. e.t.c .
The time from the injection of the sample to the peak
produced on the recorder is called Retention Time. Unknown samples are
identified based on the holding time of the known standard samples.
Gas chromatography is used to identify gases, pollutants,
foods, drugs, vitamins, pesticides, fungicides and radioactive isotopes, etc.
Steroid drugs consumed by players in international sports competitions are also
identified by this technology.
Thin Layer Chromatography
The principle of TLC is similar to the principle of paper
chromatography, instead of paper, inert substances such as cellulose are used
as supporting materials. Cellulose is spread on a glass or plastic plate as a
thin layer.
Chromatography separation in TLC is relatively rapid. In
this technique, a glass, foil, or plastic flat surface deposits such a thin
layer of fixed phase that the moving state flows rapidly without any
obstruction by the capillary action.
Analytes are also transferred along with moving from one end
to the other on this layer of moving state. The rate at which the analytes move
depends on their distribution coefficient (Kd). The speed of the analyte is
expressed by its holding factor (Rf).
Rf = Distance traveled by the analyte from its origin (DA) /
Distance traveled by the solvent from its origin (DF)
Method
Thin Layer Preparation
First make a thin solution of fixed phase and a 20 square
cm. Take a glass, foil or plastic plate and put a thin uniform layer of steady
phase on it. The thickness of the layer is based on the nature of the desired
separation. Thickness 0.25 mm for Analytical Separation. And 2 mm thickness of
the layer for Preparative Separation. After setting the layer, dry the plate.
Plate Development
This work is done in a glass tank containing 1.5 cm solvent as
a moving phase to a depth. Now cover this tank and leave it for about an hour
so that the atmosphere inside the tank becomes saturated with solvent vapor
(equilibrium).
After the equilibrium is established, remove the lid and put
it in a vertical position in the tank of thin layer plates. Separation occurs
as the cell climbs on the solvent plate (Capillary Action). Separation takes 30
to 90 minutes.
In the two-Dimensional chromatographic technique
(Technique), the analysis is first developed by placing the plate in a tank by
placing the analysis in one corner of the plate. Then, dry out the plate. Now
rotate this plate at 90 ° and re-apply the analyte and place it in a new
solvent. Placing the second solvent causes the Kd value to vary and gives us a
separate chromatograph.
Paper Chromatography
This technique was first introduced in 1941 by Martin and
Synge. Among all the chromatographic techniques known so far, paper
chromatography is the simplest technique and is used in many places.
This important work was done in 1944 by scientists named
Consden, Gordon, Martin and Synge. In recognition of this work, these scientists
were later awarded the 'Nobel Prize'.
This is a simple model of chromatography. It was invented by
Sehon Ben. In this method unknown substances are analyzed by flushing the
solvent on the Waterman filter paper. Separation of these substances is effective
due to their differential migraine.
The molecule to be separated is applied on Whatman paper.
This paper is placed in a Vessel, which contains the appropriate solvent. The
solvent is absorbed by the paper. Highly soluble substances flow rapidly with
solvent.
Other substances flow at a slower rate depending on
solubility. Also, the choice of solvent determines the rate of migration of the
substance. The separation of amino acids, nucleotides and other low molecular
weight metabolic materials is done by paper chromatography. The values set by
a solute are to be displayed as Rf-values.
Rf = distance traveled by solute / new distance traveled by
solvent.
Analyte Detection
Typically, a mixture of 25 or 50% (v / v) sulfuric acid in
ethanol is heated by spraying it onto the plate at 110 ° C to identify the
analytes, causing the compound to scorch to form brown spots.
Although the movement of compounds is based on their specific
Rf value, this method does not yield good results for identifying components.
Therefore, for identification, the speed of the compounds should be compared to
the reference compounds by TLC.
Additionally, other methods can also be identified by
placing the plates in UV light. Nowadays, several fluorescent dye-containing
thin-layer adsorbents with Fluorescent Dye are also available in the market,
using which the compound is clearly as blue, green or black spots when placed
on the plate in ultraviolet light.
By spraying specific color reagents on the plate, some
compounds are shown to be colored, such as ninhydrin, for amino acids and
peptides, so that they can be easily identified.
Thanks for publishing such great information. You are doing such a great job. This information is very helpful for everyone. Keep it up. Thanks. Vapor Intrusion Mitigation
ReplyDelete