WFI Pharmaceutical | Water for Injection in Pharma
Water for injection (WFI) is an important raw material. Usually the amount is large, so it needs to be directly prepared on-site from available drinking water. The European Pharmacopoeia, the United States Pharmacopoeia and the other Pharmacopoeias have established clear quality standards. They clearly specify the microbial limit (in CFU), electrical conductivity and total organic carbon.
The purpose of WFI Pharmaceutical:
As a solution or diluent for substances and preparations for parenteral administration
Used in large quantities for the preparation of parenteral drugs or ophthalmic drugs
For the final washing of containers (such as disposable packaging) and the production process of the above products
Pure steam is gaseous injection water used for sterilization, drying and humidification. The steam saturation, dryness and amount of non-condensable gas must be determined according to the specific application or according to the applicable regulations in EN 285.
Pharmacopoeia requirements
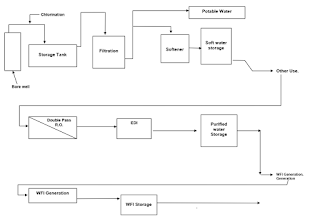
A typical WFI Pharmaceutical generation may consist of following process:
Pre-treatment Unit
Multi Grade Filters
Activated Carbon Filters
Chlorine Dosing System
First Stage RO Filtration
Second Stage RO Filtration
Antiscalant Dosing Unit
Sodium Meta Bi Sulphate Dosing Unit
EDI Unit
To read about the complete process you can read the SOP of Water System Operation.